Where are amine units found?
How Does an Amine Plant Work?
The Amine Plant 101
Now that we have established what an amine plant is and where we can find it, let’s dive into how they work. We will go step by step using a gas processing plant as our main example:
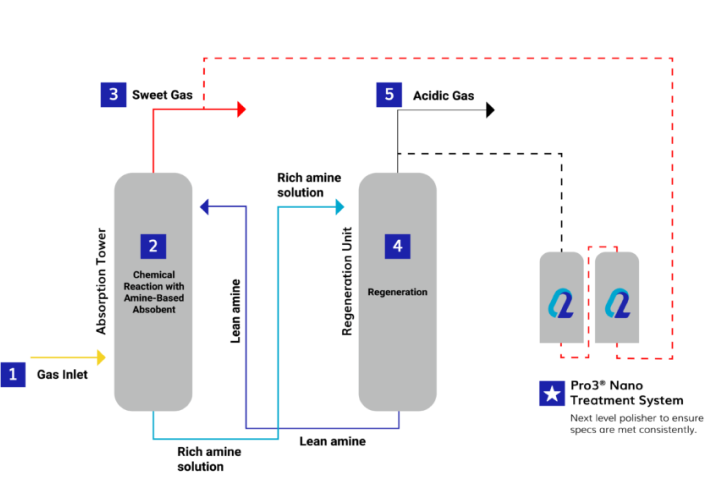
1. Gas Inlet
Raw or untreated natural gas, which contains acid gases like CO2 and H2S, enters a facility via a pipeline. In its unprocessed state, the acid gas may contain other impurities, so oftentimes the amine plant is at the front end of these types of facilities. Again, being in a raw state, this gas stream is typically saturated with water vapor.
2. Chemical Reaction
In the absorption tower, the gas comes into contact with a solution of amine-based absorbent, commonly monoethanolamine (MEA), diethanolamine (DEA), and methyldiethanolamine (MDEA). These amines selectively react with acidic gases, forming stable compounds and removing them from the gas stream. The acidic gases react with the amine solution to form soluble compounds, and because of this relation, choosing the right amine concentration is important. Due to the stoichiometric balancing during these reactions, the CO2 reaction, as it is the stronger acid of the two, the amine process is not 100% effective in stripping out all of the H2S, remaining CO2, and light mercaptans can pass unabated. In sum, amine units are moderately effective for H2S streams that have low CO2, but for weaker acids such as mercaptans, they are not a catchall and so a polisher system should be considered for final mercaptan adsorption.
3. Sweet Gas Outlet
The treated gas, now largely free of acidic gases, exits the top of the absorption tower as “sweet” or purified natural gas, ready for further processing or distribution.
4. Regeneration:
The amine solution, now rich in absorbed acidic gases, flows to a regeneration unit. Here, heat is applied to release the absorbed gases. The regenerated amine solution, now depleted of acidic gases, is recycled back to the absorption tower for reuse.
5. Recovery and Disposal:
The released acidic gases, along with any excess water vapor, are separated from the amine solution in the regeneration unit. These gases can be further processed or treated to meet environmental standards before disposal.

Source: Source Link
What Are the Key Components of an Amine Unit?
What Other Factors Should Be Considered in Amine Plant Operations?
Heat Integration: Many modern amine plants incorporate heat integration techniques to optimize energy efficiency. This involves exchanging heat between the hot regenerated amine solution and the incoming rich amine solution or between the hot gas stream and the reboiler.
Monitoring and Control: Throughout the process, various parameters such as temperature, pressure, flow rates, and concentrations are continuously monitored and controlled to ensure efficient operation and adherence to safety and environmental regulations.
Amine plants play a vital role in natural gas processing by removing the bulk of acid gases through the amine gas sweetening process, also known as natural gas sweetening process or sour gas sweetening, to meet product specifications, environmental regulations, and pipeline transmission requirements, but even with these steps, the resulting “sweet” streams may not be enough to meet commercial specifications. Remember that resulting mercaptan component after the stream had been treated? Unfortunately, mercaptans are challenging molecules to render, fortunately there are solutions.
What If My Amine Unit Isn’t Meeting Sulfur Specs?
Q2 Technologies has developed a process that takes the resulting “sweet” stream and purifies it a step further. Our Pro3® Nano and our suite of mixed metal catalysts act as a next level polisher to ensure specs are met consistently. These robust units can take swings of H2S, mercaptans, and remaining Sulfur compounds in a combined reaction and absorption process. If your amine unit is at capacity, these polishers are excellent ways to scrub out any remaining Sulfur contaminants.






