Common Questions About H2S Removal from Natural Gas Answered

Hydrogen Sulfide (H2S), commonly found in natural gas reservoirs, is a colorless gas that poses serious health and safety threats to people, including asset corrosion and degradation. Therefore, H2S removal from natural gas production processes must be an integral component of production operations; so, in this blog post, we address some frequently asked questions regarding H2S and its removal from natural gas.
What is H2S removal from natural gas?
H2S removal from natural gas refers to the practice of eliminating H2S from natural gas streams in order to prevent corrosion and meet pipeline quality standards. But first, one must confirm via appropriate test methods the levels of H2S present, see our article on test methods here. Knowing the starting point and what levels need to be achieved for safety and commercial aspects is a critical first step when considering a treatment approach for H2S.
Why is H2S removal important?
H2S is a harmful gas that can lead to respiratory illnesses, eye irritations, and other health concerns, and at high enough concentrations, it can be fatal. Furthermore, its corrosion can damage pipelines and equipment – thus making its removal from natural gas essential in protecting personnel, equipment, and the environment from damage.
How much H2S to remove?
As mentioned earlier, H2S can be fatal and lethal levels can be low as 100 ppm; therefore, many natural gas pipeline systems require thresholds of <10 ppm or sometimes <4 ppm. This is often cited in the pipeline tariffs or commercial contract and is commonly referred to as pipeline specifications or pipeline spec for short. If the tariff or commercial contract is not available, seek the lowest possible treatment threshold.
What are the methods of H2S removal?
The methods of H2S removal can be broadly categorized into two groups: commodity scavengers, and alternative scavengers. Commodity scavengers include widely used products such as MEA Triazine, Caustic Soda, and Glyoxal. These liquid scavengers, react with the H2S to form less harmful compounds through absorption or oxidation processes.
Alternative scavengers, on the other hand, may encompass products like zinc-based scavengers and MMA Triazine. While these alternatives offer effective H2S removal, they tend to be expensive or highly regulated due to their specific composition and handling requirements. This is why, within the alternative category, we can also find specialized products such as Solid Bed Catalysts and Specialized Liquid Scavengers.
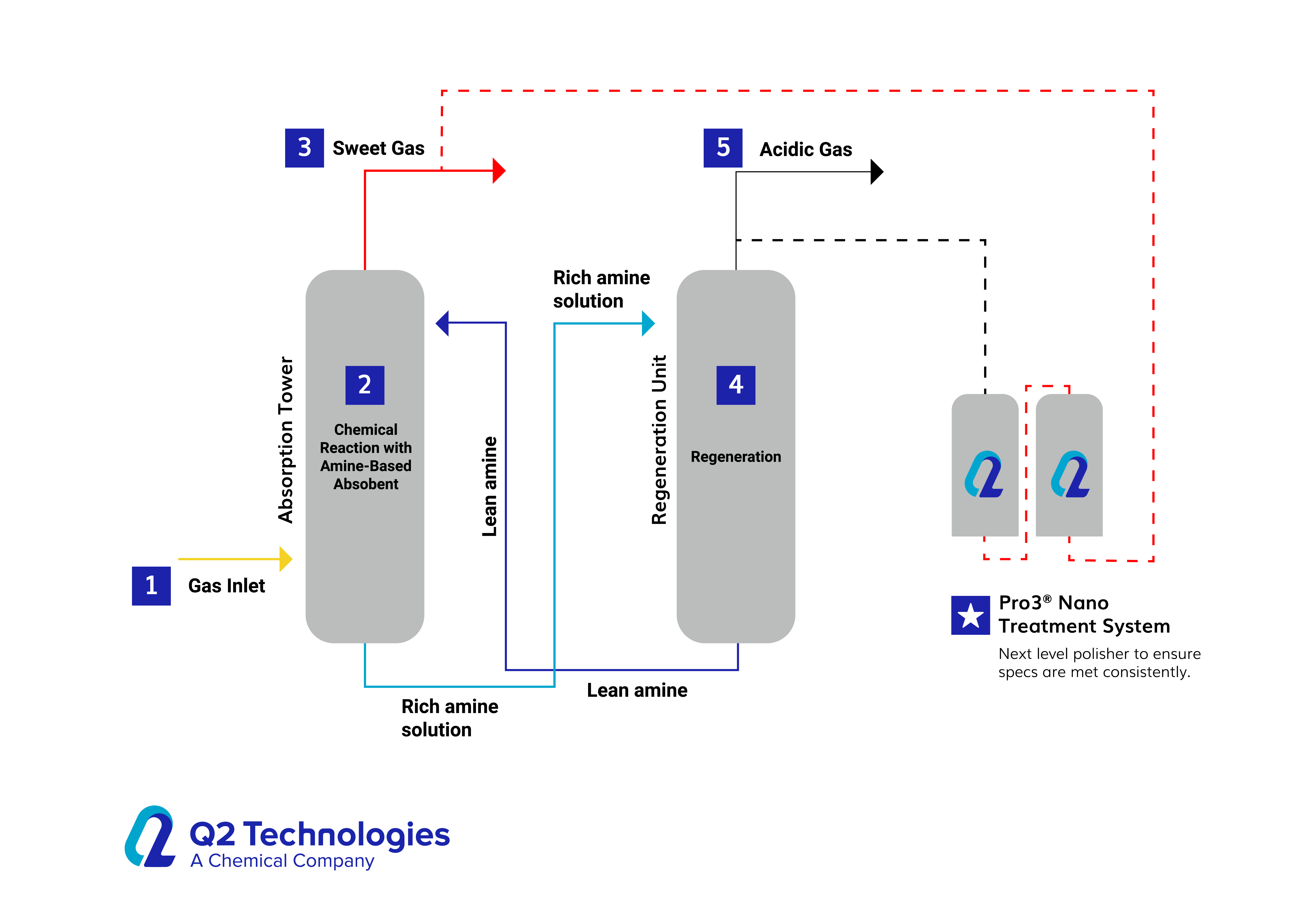
For example, there is an alternative method for H2S removal that involves the use of specially formulated metal-based catalytic absorbents called Pro3 Nano. Pro3 Nano utilizes a porous, granulated media that reacts with H2S and adsorbs mercaptans and oxygenates, providing efficient purification. This catalyst is particularly suited for gas and light liquid hydrocarbon treatment applications.
Furthermore, specialized scavengers like Pro3 GT offer a non-triazine direct substitute to traditional triazine-based products. These specialized solutions provide effective H2S removal while addressing concerns associated with triazine usage. Oftentimes, triazine needs to be closely monitored for effectiveness. For example, if over-treating of triazine occurs, scale can begin to build up and that results in blocked pipe and infrastructure, which builds up pressure creating strain on throughput. Fortunately, there are products that eliminate those concerns and still eliminate H2S.
| Commodities |
| Industry-standard products. Common liquid scavengers: |
Examples:
- MEA Triazine
- Caustic Soda
- Glyoxal
|
| Alternatives |
Specialized Solid Bed Catalysts:
Metal-based catalyst that reacts with H2S and adsorbs mercaptans and oxygenates.
Example:
|
Specialized Liquid Scavengers:
Non-Triazine direct substitute to Triazine.
Example:
|
Alternative Liquid Scavengers:
Expensive / Patented or protected products / Highly regulated products
Examples:
|
Q2 Technologies Product examples:
Pro3 Nano
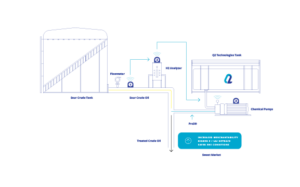
Pro3 Nano is primarily used in gas treatment applications. The combination of the catalyst and the specialized filtration in the Pro3 Nano reactor system selectively allows the passage of gas molecules while blocking the passage of larger particles and contaminants. The metal-based catalyst then allows for a reaction to take place for H2S or adsorbs mercaptans and oxygenates until the active ingredients are spent, resulting in purified gas output. The rate of consumption is calculated based on flow and inlet containment levels and is cleaned out and refilled once fully utilized. Pro3 Nano is commonly used in industries such as oil and gas, petrochemicals, and air purification, where the removal of impurities from gas streams is essential.
Pro3 GT
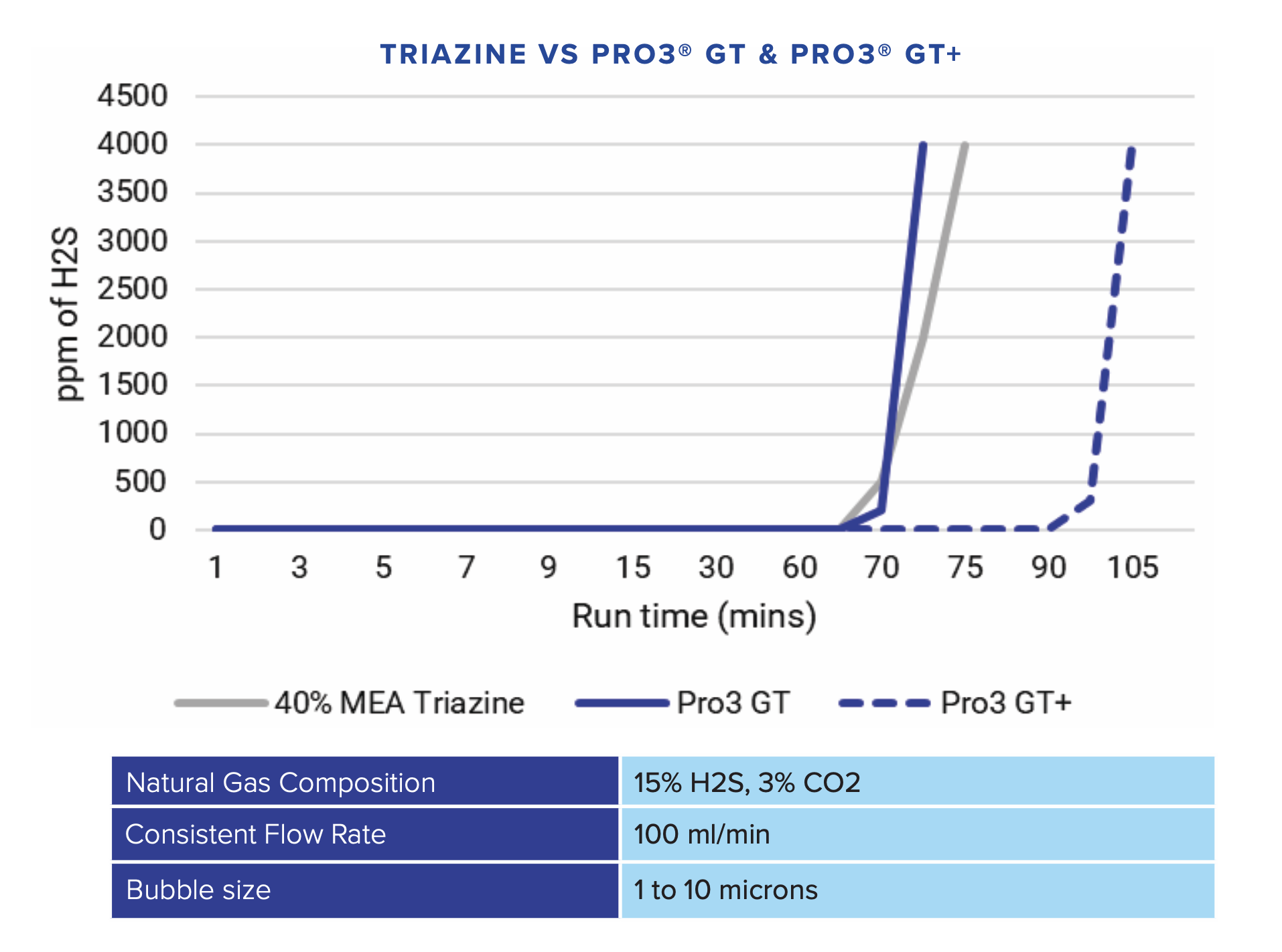
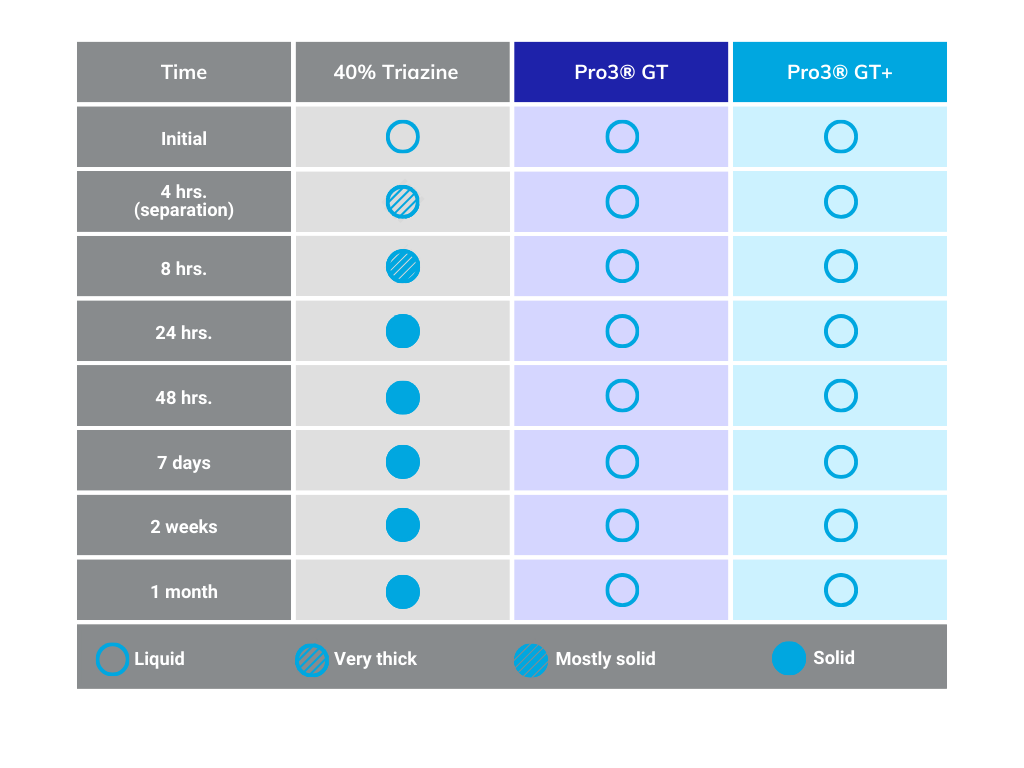
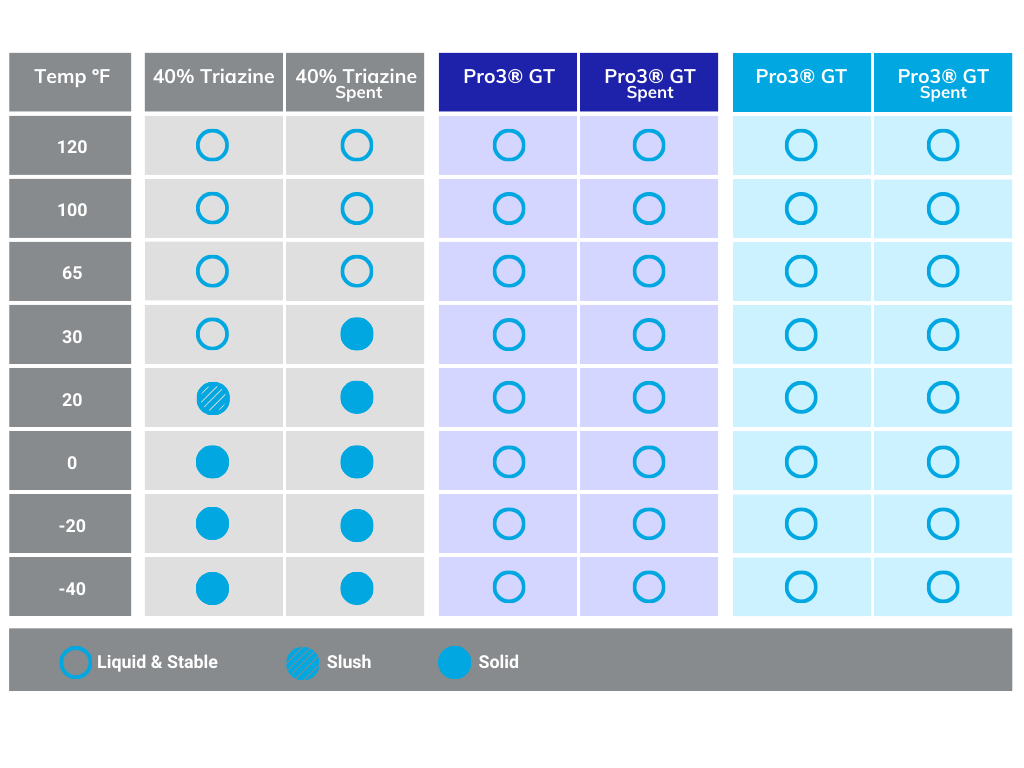
The main advantage of Pro3 GT, as compared to MEA triazine, is it does not create solids or buildup in scrubbing units; further, it offers faster reaction kinetics and increased capacity. And a hallmark benefit of Pro3 GT is its non-reversible and non-oxidizing properties when reacting with H2S.
Triazine
Triazine-based treatment has long been the commodity solution for many Producers, Midstreamers, and Refiners for many years. But now, due to advancements in technology, as mentioned above, there are a variety of alternatives that are very effective.
What is the most effective method of H2S removal?
The most effective method of hydrogen sulfide (H2S) removal from natural gas depends on several factors, including H2S concentration, desired gas purity levels, operational conditions, and economic considerations. Here are the commonly used methods and their suitability in different scenarios:
When to use commodities?
Determining when to use commodities depends on various factors. Commodity solutions are particularly valuable in situations where a facility lacks the necessary technical support or industry infrastructure to implement changes effectively. It is crucial to have personnel who are capable of consistently measuring and monitoring the progress of the commodity solution on an ongoing basis. This ensures that the desired outcomes are achieved and that the chosen commodity solution is effectively integrated within the existing framework.
When to use Metal-Based Catalysts?
Determining the appropriate circumstances to utilize metal catalysts involves considering specific factors. The iron catalyst is particularly suitable when dealing with inconsistent volume and fluctuating H2S levels. In such cases, it becomes the preferred medium due to its effectiveness in addressing these challenges. Additionally, if the operational process cannot accommodate solids and particulates as by-products, the catalyst proves advantageous. It serves as a reliable option for situations where complete filtration of H2S and its impurities is required. By employing catalysts, organizations can effectively manage these unique requirements and achieve the desired outcomes.
When to use Specialized Scavengers?
Specialized scavengers, such as Pro3 GT, come into play in specific situations when triazine has not been an effective H2S removal solution. Pro3 GT and its suite of products act as alternatives to conventional triazine-based products, addressing concerns such as no solids or particulate buildup, which unfortunately is common when overtreated or misappropriated dosing is done by triazine. By taking factors like improved performance, specific applications, and regulatory compliance into account, end-users can make informed decisions on when to opt for specialized scavengers as the most appropriate choice for their H2S removal requirements.
How is H2S removal measured?
Measuring H2S removal in natural gas involves assessing the concentration of H2S before and after the removal process. This measurement is typically expressed in parts per million (ppm*), which indicates the number of H2S molecules per million molecules of natural gas. The objective of H2S removal is to achieve a concentration that complies with pipeline quality standards and ensures the safety of personnel and equipment.
*In some instances, one may see ppm expressed as ppm/v or ppm/w. The added “/v” and “/w” indicate “by vapor” or “by weight”, respectively. The vapor reading simply indicates the ppm level in the headspace of a specific volume, and the by weight (also known “by liquid”) is measuring the ppm in the liquid portion of the volume. Therefore, when measuring H2S in natural gas, measuring the vapor phase of ppm is most common.
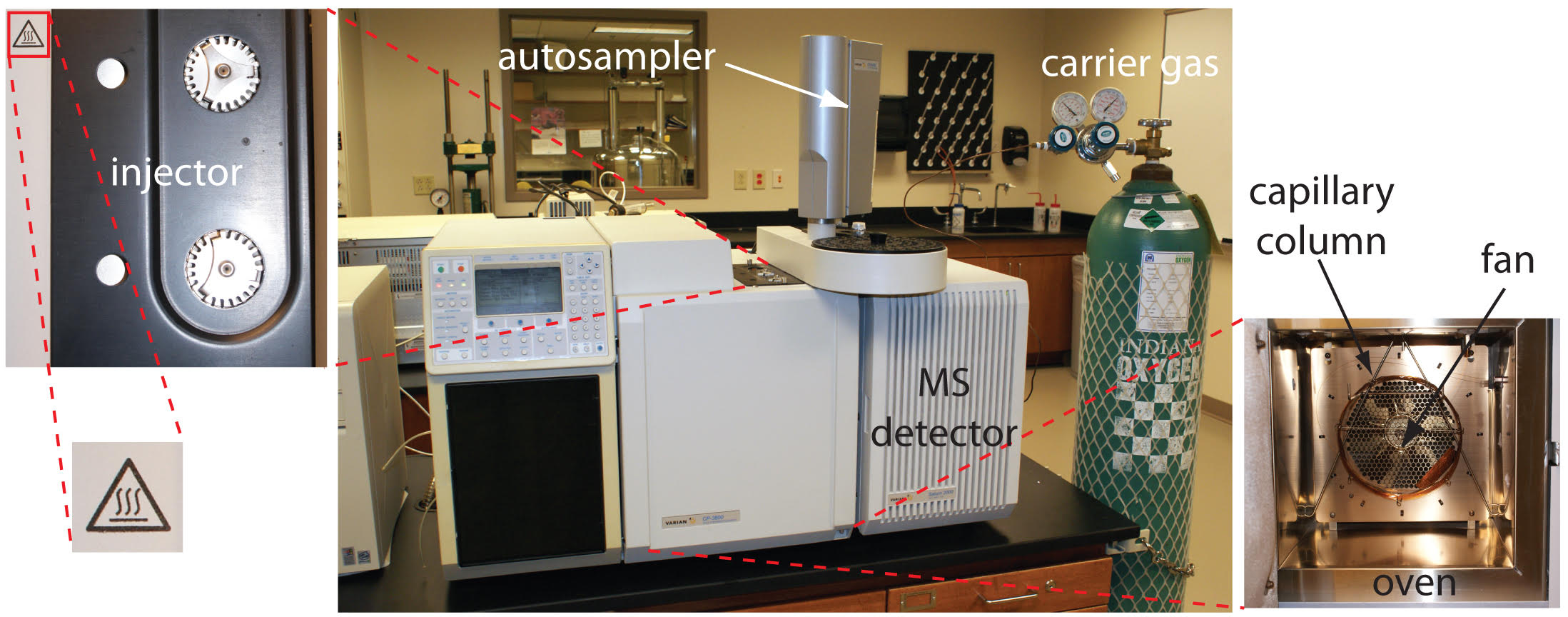
To determine the effectiveness of H2S removal, various industry-approved test methods are employed. These methods help quantify the H2S concentration accurately:
- Gas Chromatography (GC): It separates and quantifies H2S from gas mixtures by analyzing its retention time or peak area.
- Electrochemical Sensors: These devices detect H2S concentration based on its electrochemical reactions, measuring current or potential.
- Titration Methods: They determine H2S concentration by reacting it with a known reagent that generates a measurable signal or color change.
- Spectroscopic Techniques: These techniques use light interaction to detect H2S concentration through its absorption or emission at specific wavelengths.
What are the safety considerations when removing H2S from natural gas?
H2S removal from natural gas is a hazardous process that requires proper safety measures to be in place. Safety considerations include:
- Always review safety protocols when on location before starting any work around natural gas.
- Personal protective equipment (PPE): Personnel should wear appropriate PPE, such as respiratory protection, chemical-resistant clothing, and eye protection to prevent exposure to H2S and other chemicals.
- Ventilation: Adequate ventilation should be provided to prevent the buildup of H2S and other gases in the work area.
- Monitoring: Gas detectors and monitoring equipment should be used to continuously monitor the work area for H2S and other hazardous gases.
- Emergency response: Emergency response procedures should be in place in case of accidental exposure or release of H2S.
What are the environmental considerations when removing H2S from natural gas?
When removing H2S from natural gas, several environmental considerations need to be considered. One crucial aspect is the proper handling of waste and emissions generated during the process.
To handle emissions, it is crucial to implement appropriate control measures. These include regular inspections, well-maintained equipment, and leak detection systems. By promptly identifying and addressing any potential leaks or releases, the emission of H2S or other hazardous gases, such as SO2, can be minimized.
Once the H2S removal process is successfully completed using a scavenger such as triazine or Pro3 GT, the next important step is to address the management of the spent product. For these types of products, the commonly employed method is sending it to a Saltwater Disposal well (SWD). SWDs involve treating the spent triazine solutions to eliminate impurities before responsibly disposing of them. This approach aims to minimize any potential environmental impact and adhere to waste management regulations.
However, when dry media scavengers like Pro3 Nano are used, waste handling techniques vary slightly. These scavengers typically produce solid waste in the form of spent catalyst. Proper waste management involves careful collection, packaging, and transportation of the spent media to a designated landfill facility equipped to handle and contain hazardous materials. By following this procedure, the waste can be appropriately disposed of in landfills as the spent material is non-hazardous passing TCLP tests, responsibly sending it to landfills reduce the risk of environmental contamination.
What are the benefits of H2S removal from natural gas?
The benefits of H2S removal from natural gas include:
- Improved safety: H2S removal prevents health and safety hazards associated with exposure to H2S and corrosion of equipment and pipelines.
- Meeting pipeline quality standards: H2S removal ensures that natural gas meets pipeline quality standards and can be transported safely and efficiently.
- Environmental protection: H2S removal minimizes the environmental impacts associated with natural gas production and transportation.
Who provides H2S removal services?
At Q2 Technologies, we specialize in providing natural gas treatment solutions, including H2S removal services. With years of experience in the industry, we have the expertise and advanced equipment necessary to design, implement, and optimize H2S removal systems for various natural gas streams. If you are in need of H2S removal services for your natural gas operations, trust Q2 Technologies as your reliable and experienced partner. We have a proven track record of delivering effective solutions tailored to your specific needs. With our expertise, commitment to innovation, and focus on customer satisfaction, we stand out as a leading provider in the field of H2S removal services.