Not all Hydrogen Sulfide (H2S) is created equal! But it results in the same damaging, toxic, and deadly gas. Knowing the source can ultimately assist in finding the appropriate treatment for a permanent solution. In today’s blog we’ll discuss the two major ways H2S in the oil and gas industry can come about, the differences between the two formations, and maybe some uncommon ways to approach finding a solution to these problems.
Hydrogen sulfide in crude oil and gas (H2S) is commonly present in reservoirs, posing both challenges and opportunities for the industry.
Why Understanding H2S Formation is Crucial for Oil & Gas Operations
What are the two faces of H2S formation
Biological Formation
This process involves the breakdown of organic matter (think buried plant and animal remains) by sulfate-reducing bacteria (SRB) in oxygen-depleted environments. These bacteria utilize sulfate (SO4²⁻) as an electron acceptor during organic matter degradation, releasing H2S as a byproduct. This mechanism is prevalent in formations with past or present sulfate-rich brines and organic material.
A few great examples of biological formation of H2S can be found in swampy bayous, peat bogs, stale pond water, or uncirculated retention ponds at oil and gas or wastewater treatment facilities.
Geological Formation
Here, H2S generation doesn’t involve living organisms. It can occur through:
- Thermal Decomposition: High temperatures can break down sulfur-containing minerals like gypsum (CaSO4·2H2O) into H2S, calcium oxide (CaO), and water vapor.
- Thermochemical Sulfate Reduction (TSR): Under high temperatures and pressures, reactions between hydrocarbons and sulfate minerals can yield H2S, carbon dioxide (CO2), and metal sulfides.
Geological creation of H2S in oil reservoirs is happening at major fault lines all around the world, especially where uplifts are grinding against active oil formations. A well-known example is the eastern slope of the Delaware as it is slowly being lifted by the Central Basin Platform, a granite uplift that bifurcates the two major lobes of the Permian. This movement allows for the geological process to occur deep underground in the slightest alcoves of rock fissures and micro pore spaces.
How to determine where H2S is coming from?
There’s a technique called EA-IRMS that helps us determine where sulfur comes from. Here’s how it works:
Like a Fingerprint:
Sulfur has a unique “fingerprint” depending on where it originated, either from deep underground (geological) or from living organisms (biological). EA-IRMS analyzes these tiny variations in the sulfur atoms to differentiate between the two sources with the δ34S scale (colloquially pronounced delta 34 S). The δ34S scale is a way for scientists to measure the relative abundance (“fingerprints”) of two types of sulfur atoms: sulfur-32 (³²S) and sulfur-34 (³⁴S). By measuring the δ34S value of a sample (compared to the CDT reference), scientists can gain insights into whether the sulfur originated from geological processes or was influenced by biological activity.
Interpreting the δ34S Values:
- A δ34S value greater than 0 on the scale (positive) suggests that bacteria have been using up δ32S, leaving behind a higher proportion of δ34S. This indicates a likely biological influence.
- More negative δ34S values suggest that there has been little to no biological activity using up δ32S. This might point towards a more geological origin of the sulfur.
Let’s take a look at this example:
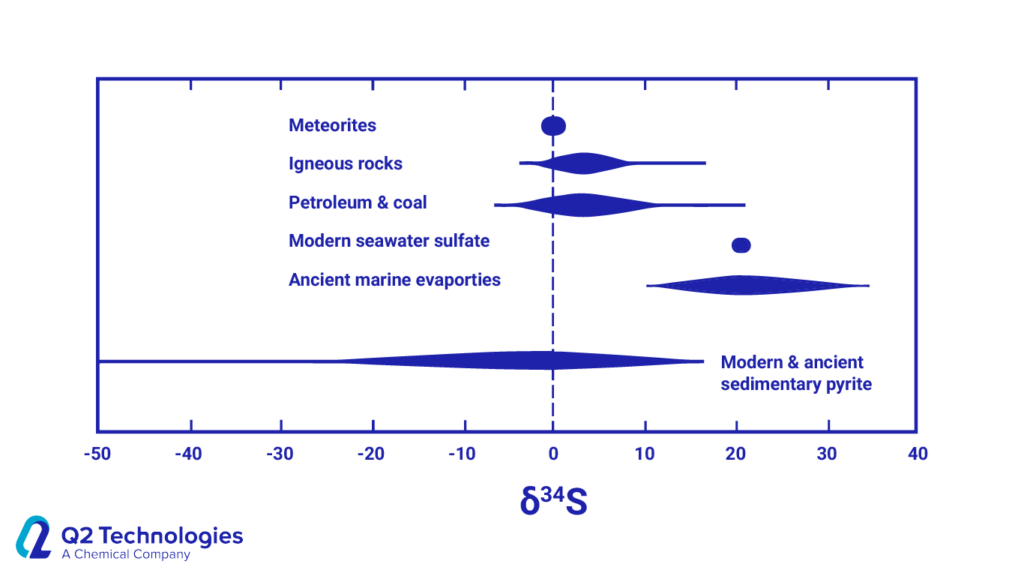
Source: Seal II RR (2006). “Sulfur Isotope Geochemistry of Sulfide Minerals”
- High δ34S values (positive side): Reservoirs like modern seawater sulfate and ancient marine evaporites often show higher δ34S values. This suggests a possible biological influence from sulfate-reducing bacteria.
- Intermediate δ34S values: Meteorites, igneous rocks, petroleum, and coal might have some biological influence due to their position on the positive side of the scale. However, their values might not be as extreme as seawater sulfate, indicating a potential mix of geological and biological processes.
- Low δ34S values (negative side): Modern and ancient sedimentary pyrite tend to have lower δ34S values, suggesting a more dominant geological origin with less biological influence.
The Takeaway
Getting to know the formation mechanism and age of H2S in your reservoir provides significant advantages in several areas. From a safety standpoint, understanding its origin allows you to predict H2S concentration and implement appropriate safeguards for drilling, production, and transportation. Furthermore, knowing the formation mechanism guides the selection of the most efficient H2S removal techniques for your produced oil and gas streams. Finally, the presence and type of H2S offer valuable clues into the geological history and properties of the reservoir, aiding in accurate reservoir characterization.
Explore our H2S scavengers.







