What is a mercaptan?
A mercaptan also known as a thiol is a sulfur compound that is naturally occurring in both crude oil and natural gas. It is the sulfur equivalent of an alcohol and comes in the form an R-SH, where R represents an alkyl or other organic group. What is interesting about mercaptans is that unlike its cousin (H2S/hydrogen sulfide) it can come in many different forms – methyl mercaptan, ethyl mercaptan, propyl mercaptan, butyl mercaptan and other branched and more complicated variations. Interestingly, we have all been exposed to mercaptans as its used as an odorant additive in commercially sold natural gas so that people can easily detect a leak through smell. Figure 1 shows the Methyl Mercaptan molecule and Figure 2 shows the Ethyl Mercaptan molecule.
Figure 1. Methyl Mercaptan molecule Figure 2. Ethyl Mercaptan molecule
How are mercaptans measured in crude oil?
The most common way to measure mercaptans in crude oil is through two standardized test methods- ASTM D3227 and UOP 163. Both methods can be run at oilfield or inspection laboratories. Both test methods are titration based and require lab equipment. We have seen costs for these tests to range between $250-$500. The results obtained are in parts per million by weight (ppmw) and reported as mg/kg. It is important to note that chemical contaminants in crude oil may affect the results obtained through these test methods. Unlike H2S that can be measured in both the vapor phase (parts per million by volume) and ppmw, both test of these methods only yield a ppmw result.
More detailed analysis on mercaptans can be done through sulfur speciation studies as shown on Figure 3.
How do you remove mercaptans from crude?
At Q2 Technologies we regularly remove mercaptans from crude oil and condensate using both our Pro3® and ProM®chemistries. By removing mercaptans we help producers meet pipeline requirements and help traders meet contract specifications for crude sold to refiners. We typically see mercaptan requirements in pipeline tariffs to be less than 50 ppm. In early 2020 we successfully treated a 750,000 barrel export cargo with high mercaptans.
Treating mercaptans is more complicated than H2S. Mercaptans are heavier and more complex molecules that require more time and contact for the chemical reaction to happen. We recommend to performing lab testing prior to chemical treatment to ensure specifications will be met. Figure 3 below shows a sulfur speciation done before ProM® and post ProM® treatment. To find out about the materials and methodology we followed to obtain the data and to learn how we can help you remove mercaptans from your crude, visit our detailed guide on removing mercaptans while keeping costs in line or contact us today.
Sulfur Speciation Pre and Post ProM® Treatment
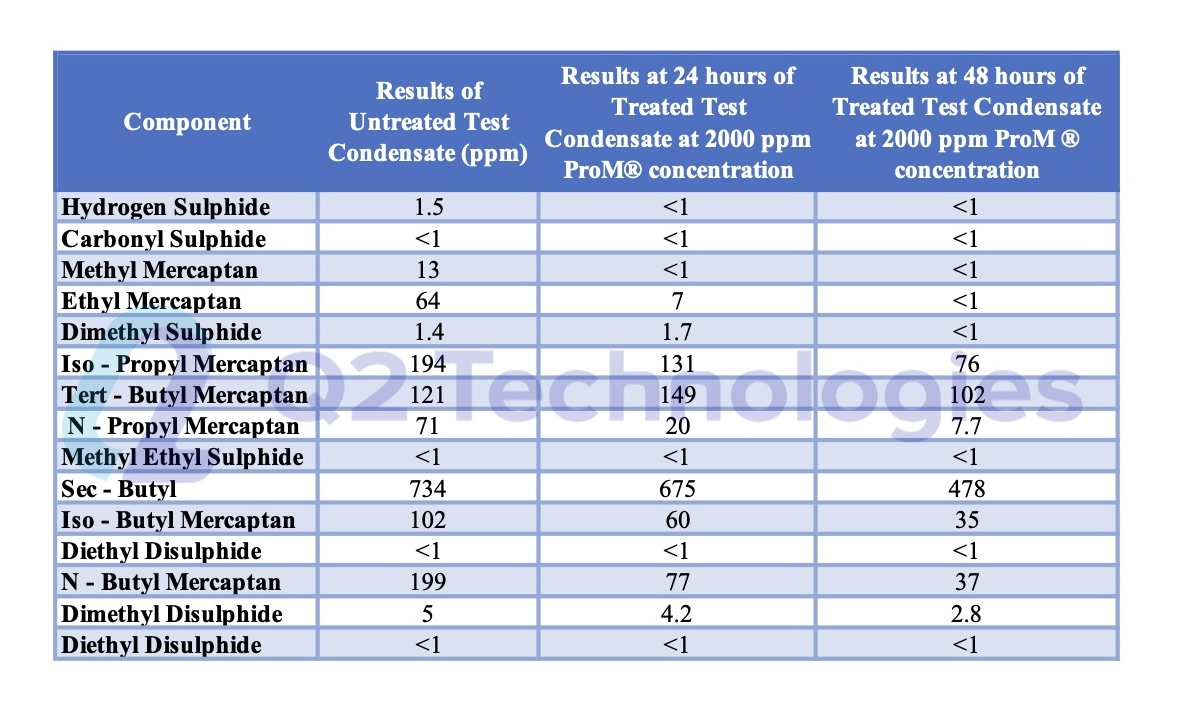
Figure 3. Sulfur Speciation Pre and Post ProM® Treatment







