Why Choose Between Triazine and Non-Triazine?
When selecting an H2S scavenger whether a traditional triazine-based product, an alternative triazine formula, or a non-triazine solution—it’s important to carefully evaluate all available options. Different scavengers perform better under specific process conditions and applications, such as oil treatment, acid gas sweetening, or mercaptan odor removal. As our analysis will show, one may be better than the other depending on the specific conditions of what needs to be treated. Over the years, triazine-based treatment has been the go-to solution for many Producers, Midstreamers, and Refiners, and to a large extent it is still the go-to choice. But for some, implementing new technology such as non-triazine products has positively impacted their operations and has lowered their costs. Is one better than the other? That all depends on your use case and process conditions. Here you’ll find a brief description of both triazine and non-triazine alternatives to help inform you of the effective solutions on the market for your application.
| Aspect | Triazine Treatment | Non-Triazine Treatment |
|---|---|---|
| Industry Usage | Longtime go-to solution for Producers, Midstreamers, and Refiners | Gaining adoption as new technology |
| Reliability | Proven and still widely used | Effective alternative depending on conditions |
| Impact on Operations | Trusted but may have operational challenges (e.g., fouling, amine salts) | Can positively impact operations, often lowering costs |
| Best Use Case | Suited for many traditional applications | Better fit in certain cases where triazine limitations matter |
| Key Consideration | Still the default choice for many | May be preferred when cost savings or refinery safety is a concern |
What is Triazine Treatment?
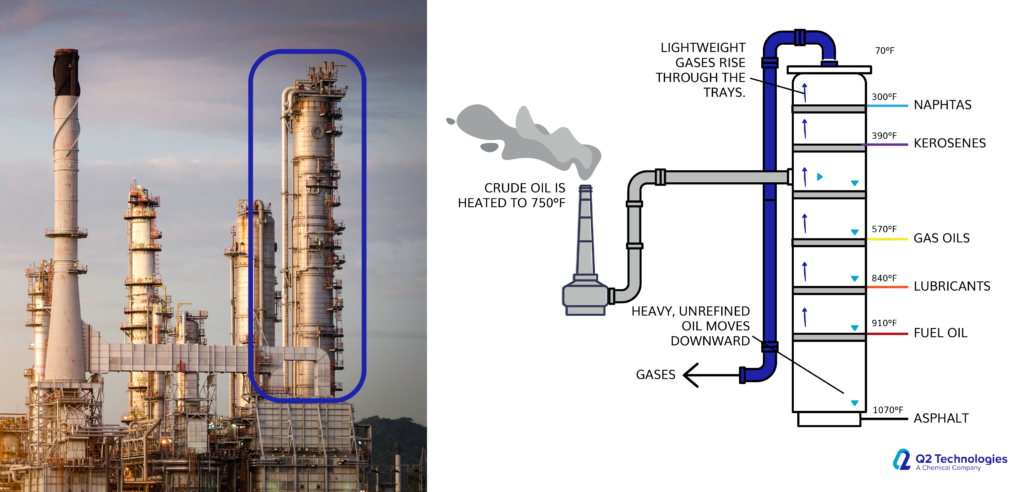
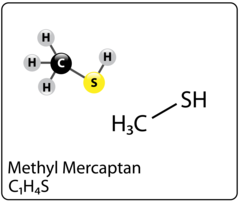
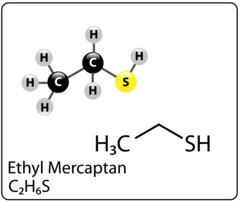
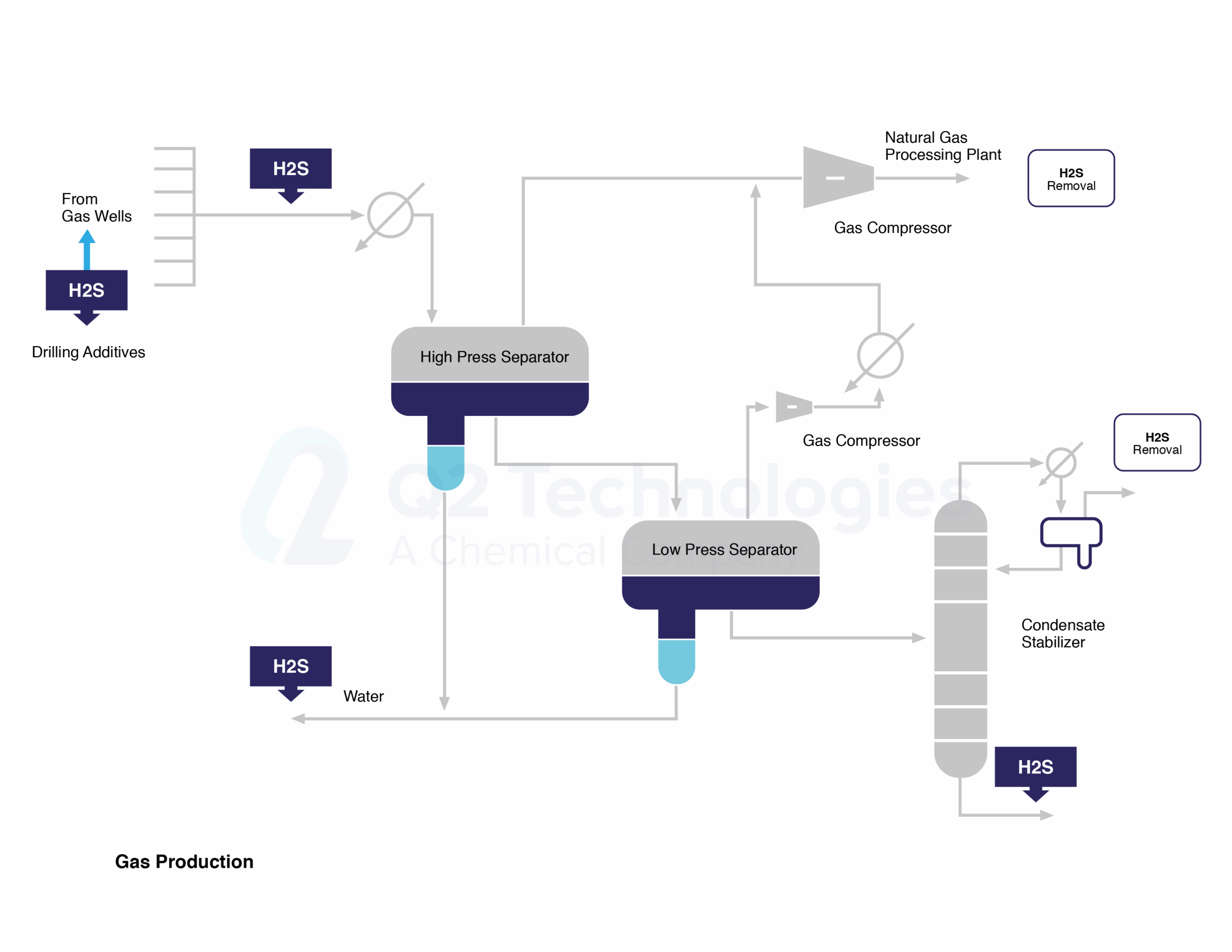
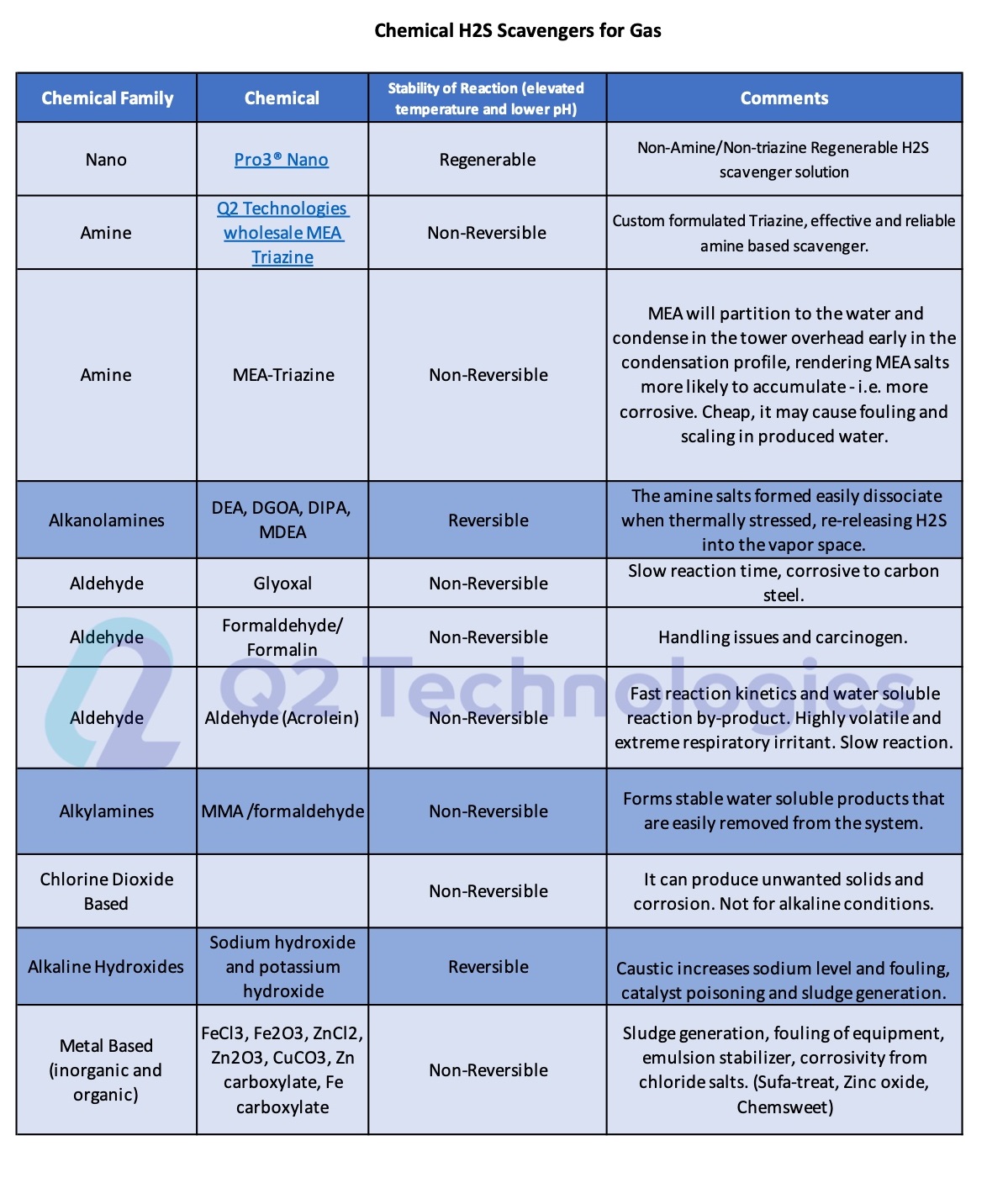
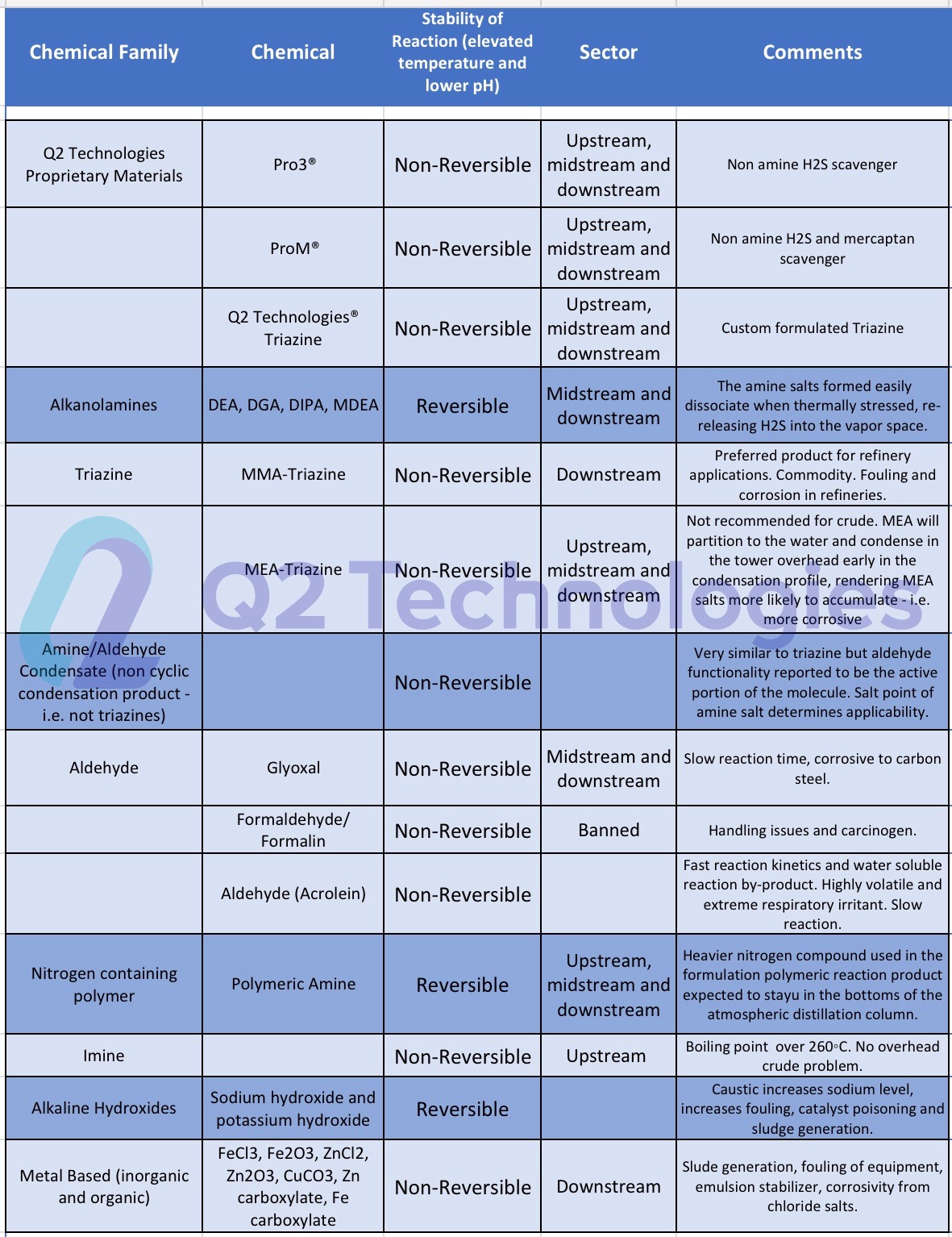
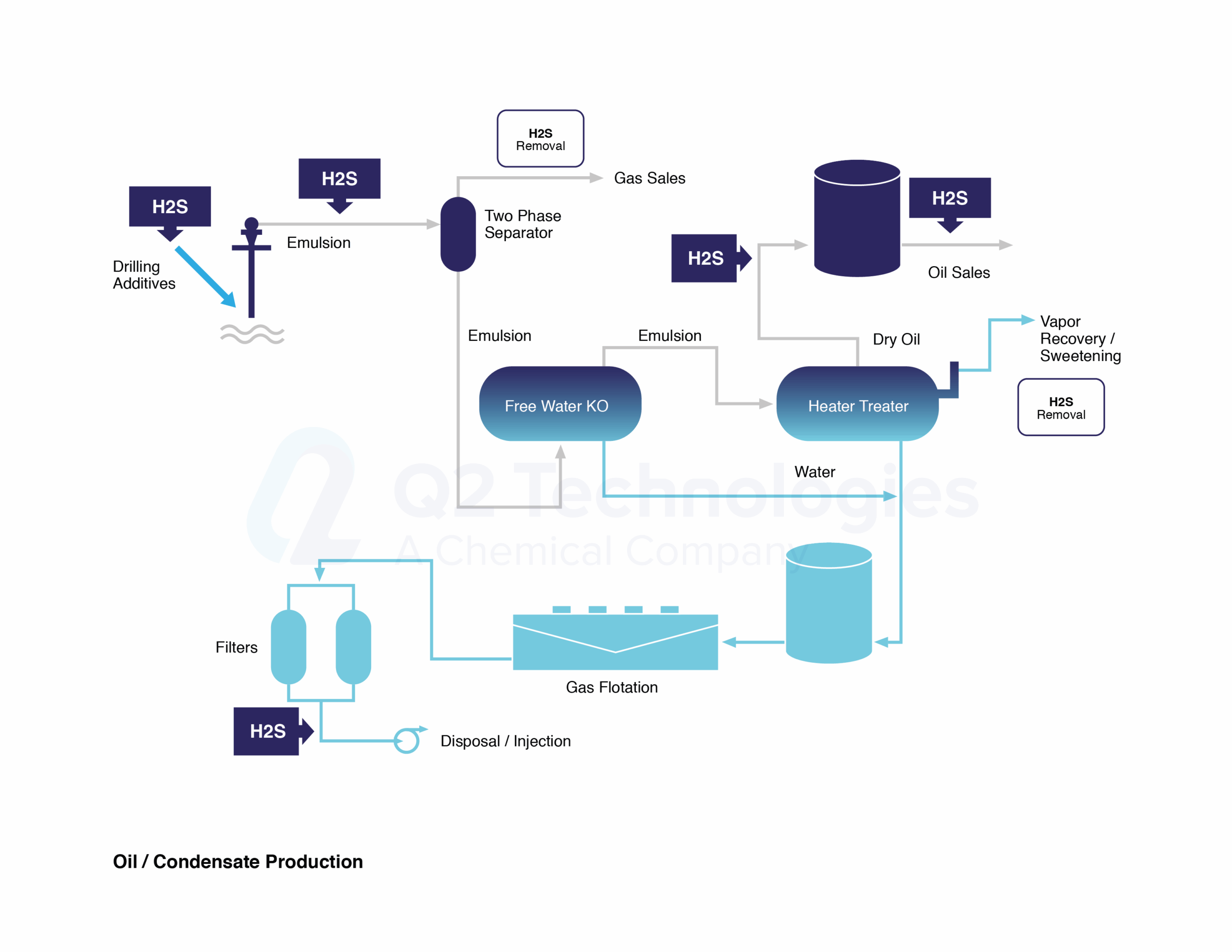
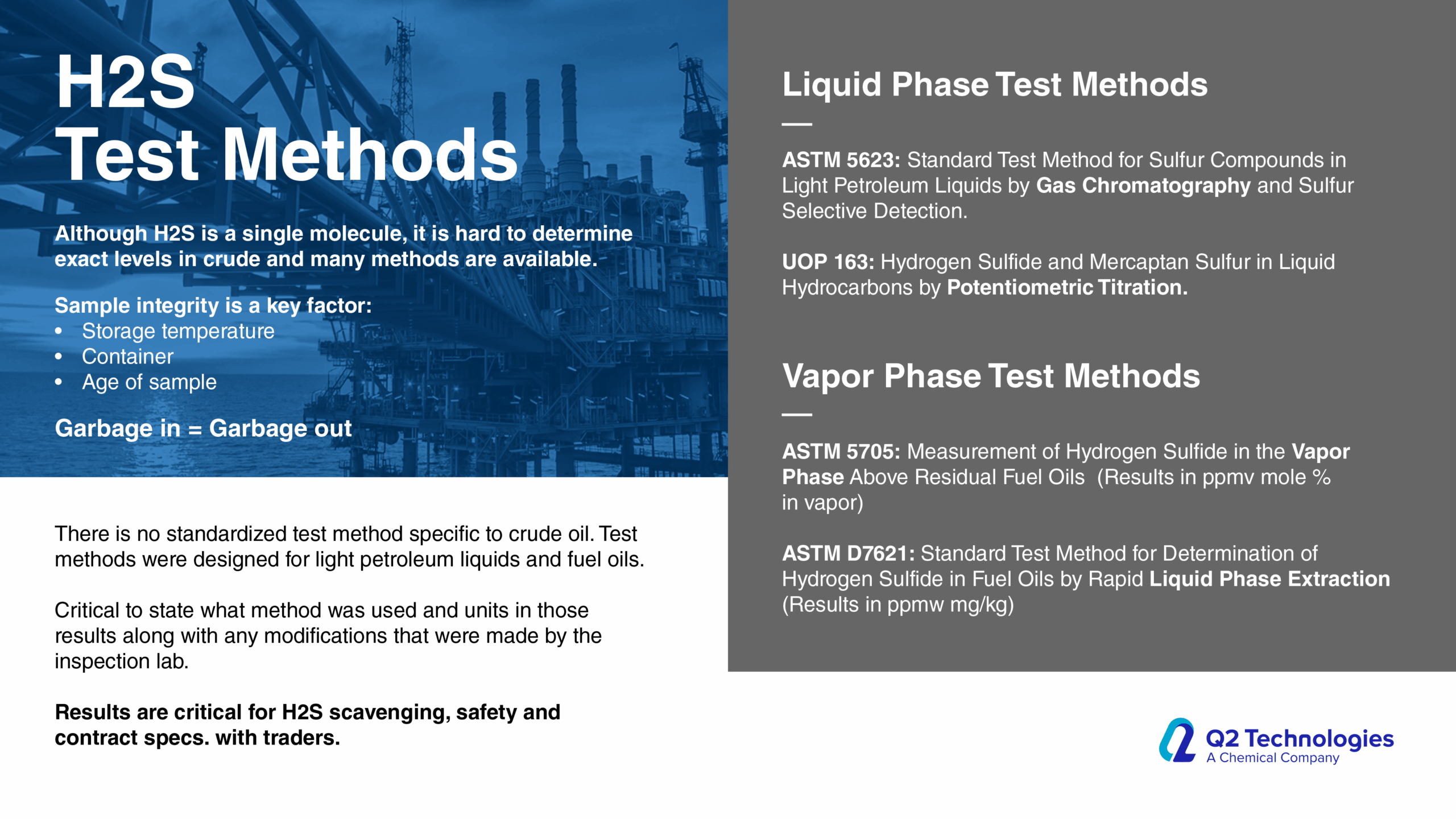
Triazine for H2S scavenging is an established and reliable chemical reaction that is employed in the Oil & Gas industry to remove H2S from gas streams. The H2S scavenging can be done via direct injection in pipelines with fog nozzles, wet scrubbers, or bubble towers at either wells, central batteries or midstream systems. The triazine H2S scavenger technique is a cost-effective solution for gaseous streams with sulfur loading of less than 1000 lbs. per day. However, consider alternative catalyst systems for extended long-term use and savings. It typically reduces H2S concentrations to <4 ppm and partially removes several light mercaptans (methyl, ethyl, and propyl). Triazine’s ability to remove H2S is affected by various parameters, including temperature, pH, contact duration, and natural gas composition. The final product is water soluble and is usually discharged in the produced water tanks and commercial saltwater disposal facilities.
It is important to note that using triazine as an H2S scavenger may cause fouling from the polymerization of dithiazine in contact towers, scrubbers and/or pipelines. Careful use and monitoring of the triazine solution is needed to ensure smooth operation. Q2 Technologies has been manufacturing traditional triazine blends for decades and also offers expertise in developing unique formulas for special cases.
What is Non-Triazine Treatment?

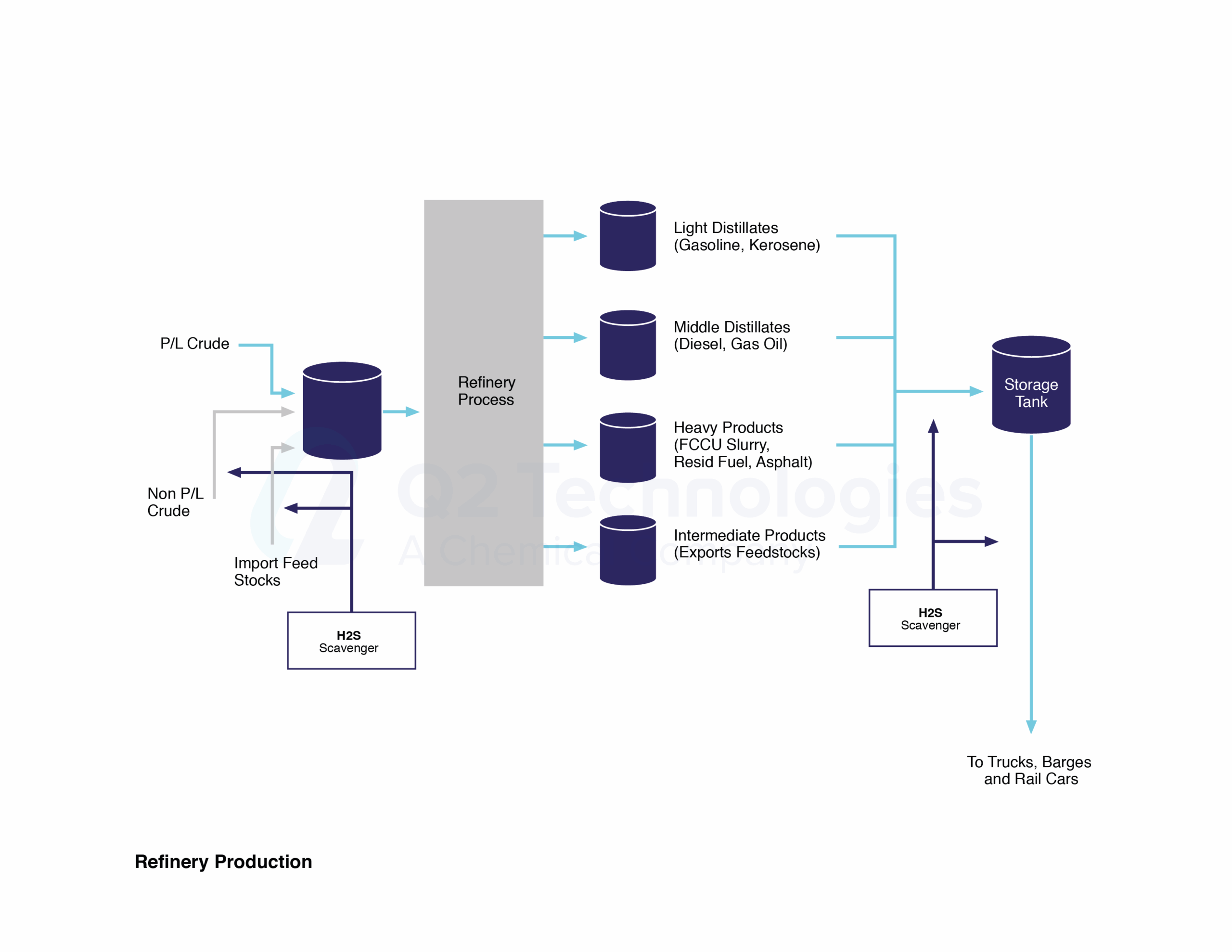
Even though triazine is a well-trusted solution, many companies have chosen non-triazine-based scavengers as alternatives for use in crude oil applications. One of the main characteristics of our non-triazine product, Pro3®, is a non-amine and non-glyoxal technology, making it safe for refineries and pipelines. If left unchecked, Amine salts that form from spent triazine can ultimately cause damage to the top stacks of a refinery when heated to >500 °F. Many refiners in the US Gulf Coast and overseas monitor amine content and require traders and midstream companies to use vetted non-triazine chemistries.
Advantages of Non-Triazine H2S Scavengers
Producers and Midstream companies alike have found success with our non-triazine products:
- The quality of the oil is upgraded when H2S content is reduced.
- Safer environment for all personnel on-site.
- Infrastructure and equipment will last longer now that corrosion caused by H2S has been neutralized.
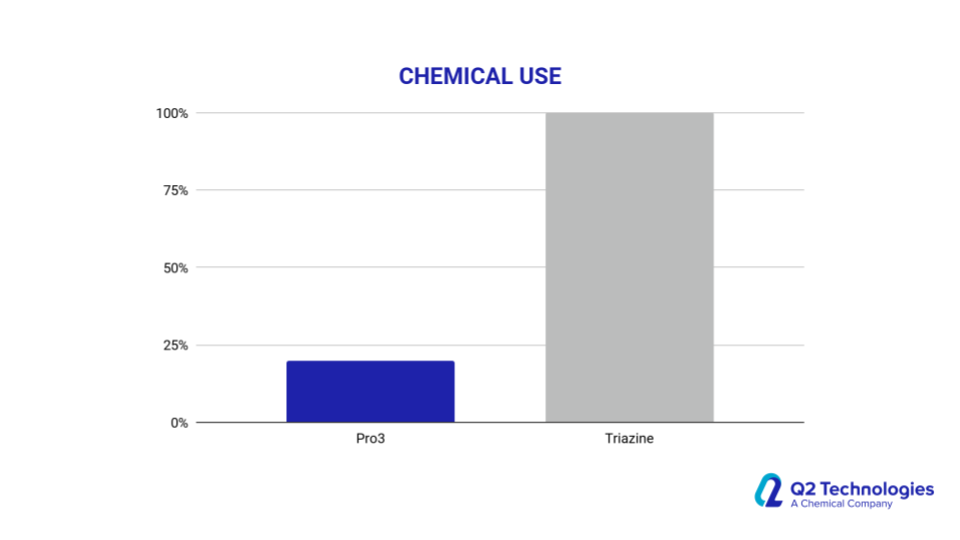
- Our non-triazine scavengers are not based on commodity-priced products, so our prices are protected and are less impacted by external factors such as freezes and industrial raw material shortages.
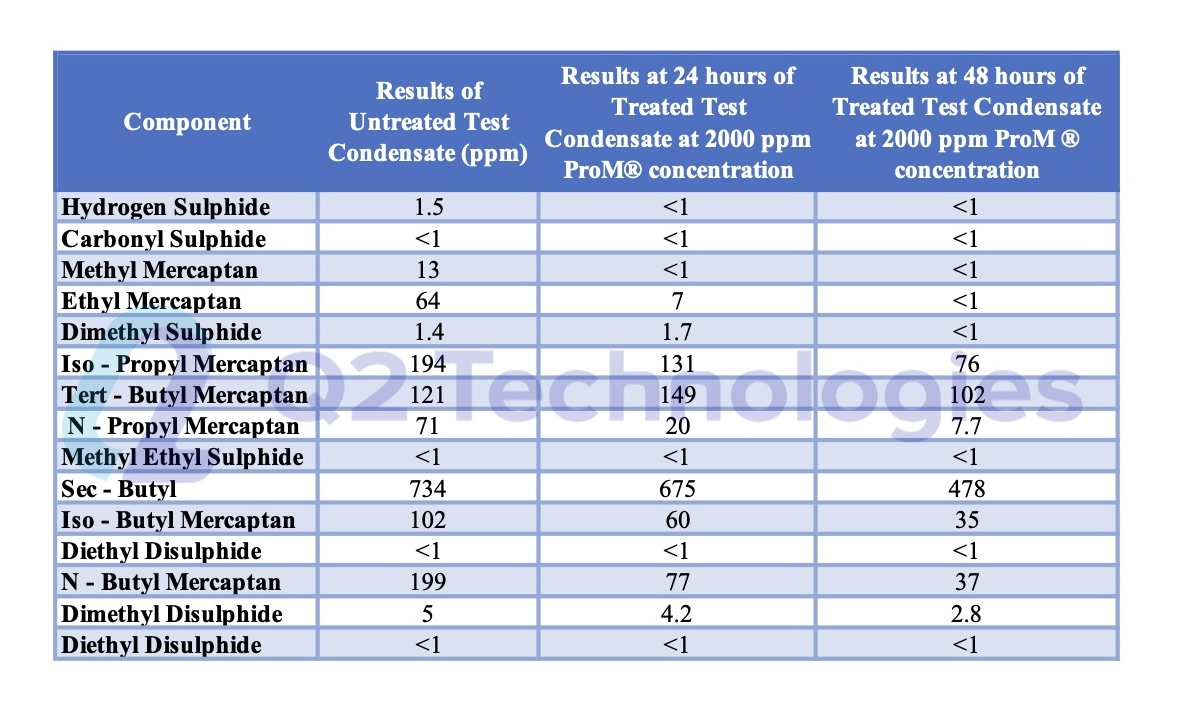
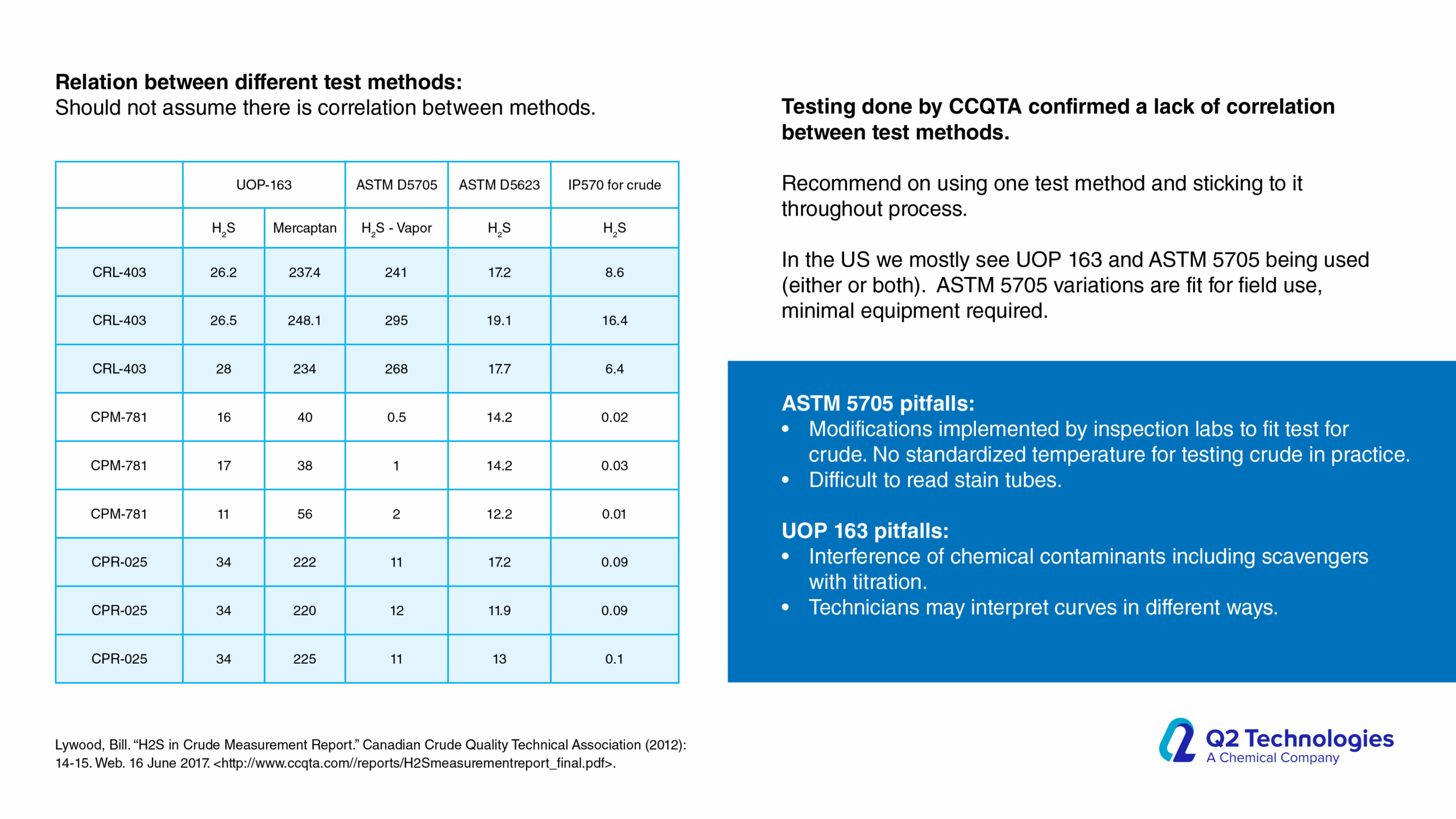
Field Results and Performance
Triazine vs. Non-Triazine: Key Considerations
In conclusion, both triazine based H2S scavenger and-non triazine H2S scavenger options have their benefits and are the go-to alternatives for oil and gas treatment. However, one may be better than the other depending on the specific characteristics of the product that needs to be treated. For more information on triazine-based scavengers for natural gas operations, check out our detailed overview here. If you’re looking for the right H2S scavenger solution for your company, feel free to contact us! You can also read more about how switching to Pro3® can be the game-changer for your operations in our detailed comparison of triazine versus non-triazine treatments here
Sources:
https://www.sciencedirect.com/science/article/abs/pii/S1875510017304559