Read more blogs from this series:
- Part 1
- Part 2

Real-World Evidence of Scale Formation
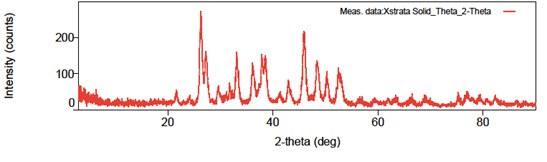
A sample of these scales was analyzed with XRD, and the results (shown in the graph below) indicate the presence of calcium carbonates and strontium carbonates. The height of the peaks corresponds to the concentrations of these compounds, with higher peaks indicating higher concentrations.
Causes of Scale on operations
This is by no means an exhaustive list, but scale can be caused by the following:
- Decreased oil production
- Increased water production
- Influx of contaminants or formation sand
- Plugged tubing strings
- Plugged perforations
- Stuck or leaking valves
- Plugged gas anchors
- Blocked flow lines
- Fouled fire tubes
- Heaters and heat exchangers offline
- Cooling tower basins
- Damaged boiler tubes
- Fouled dump valves
- Mismanaged metering equipment
- Cemented filters
- Downtime for maintenance
…Just to name a few potential headaches!
Removing existing scale:
Why Scale Must Be Removed Promptly
Key reasons for removing scale include:
- Production may be adversely affected by a scale layer.
- Corrosion is accelerated under a scale layer.
- Bacteria grow and thrive under a protective scale layer.
- Scale formation accelerates when seated by existing scale. In other words, scale will form quicker in dirty systems than in a clean system. Scale begets scale.
How to Prevent and Treat Scale Formation in Oil Fields?
Always consult a scale or chemical specialist before doing any work that could modify the physical or chemical properties of an asset.
Preventive measures
- Careful monitoring and management of the chemical properties of the water.
- Various treatments can be used, including chemical inhibitors, mechanical removal, and adjustments to water injection rates and chemistry. The specific approach will depend on the characteristics of the formation water, injection water, and the oil reservoir itself.
Treatment methods
- Chemical inhibitors: often used to prevent scale formation by modifying the chemistry of the water to prevent minerals from forming solid deposits.
- Mechanical removal: scraping or jetting, to remove scale deposits from equipment.
- Adjustments to water injection rates and chemistry may also be made to prevent the mixing of incompatible waters.
Acid Treatments for Scale Removal
There are two major types of acids used for breaking up scale: mineral acids, which are inorganic, and organic acids, which contain a carbon component.
Mineral Acids:
- Hydrochloric acid, most common (HCl)
- Hydrochloric + Hydrofluoric acid (Mud acid)
- Sulfuric acid (H2SO4)
- Nitric acid (HNO3)
- Phosphoric acid (H3PO4)
Organic Acids:
- Acetic Acid (CH3COOH)
- Formic acid (HCOOH)
However, it’s not simply a matter of pumping acid down a well and expecting all scale to dissolve. Proper dissolution requires the acid to effectively contact the scale. Three main challenges are typically encountered:
Challenges:
- Pumping acid to scale sites.
- Ensuring acid potency over distances.
- Analyzing scale composition for effective treatment.
However, it’s not simply a matter of pumping acid down a well and expecting all scale to dissolve. Proper dissolution requires the acid to effectively contact the scale. Three main challenges are typically encountered:
It’s a Wrap
Now that you’ve gained insight into oil field scale, you’re better prepared to engage with operations personnel about scale formation. For a deeper dive into pipeline challenges and solutions, check out our full article here
For advanced solutions in chemical applications, Q2 Technologies leads in H2S and mercaptan removal.
Sources
https://www.scirp.org/journal/paperinformation?paperid=94662
https://beyerplumbing.com/wp-content/uploads/limescale-1-scaled-e1650317941895.jpeg
https://html.scirp.org/file/3-1020683×15.png
Company photos and data, confidential.







