Read more blogs from this series:
- Part 1
- Part 2

Scale formation at a pipeline facility, causing significant downtime and increase in maintenance expenditures. Scale formation can happen along many parts of a system. Continue reading to learn more!
What is Scale?
To start, scale is the accumulation of mineral deposits that form when incompatible water sources and dissolved minerals come into contact. This usually happens under ideal conditions on the surfaces of pipes and other equipment in oil and natural gas operations. Scale can appear early in the production cycle, near the wellhead or at a tank battery, and can migrate downstream, affecting midstream facilities and terminal stations.
Why is scale a concern?
All scales have the potential to cause massive operational and safety issues in the oil field, such as:
- Reduced efficiency of equipment
- Decreased flow rates
- Potential line breaks and serious damage
While maintenance requires attention and resources, the unchecked impact of scale formation can result in significant financial losses.
How is scale formed?
Scale forms when water mixed with minerals creates solid deposits. In oil fields, this typically involves:
- Formation water: Water that is naturally present in the production horizon of oil-bearing and natural gas reserves.
- Injection water: Water and components injected into the formation to stimulate oil and gas production.
When these two types of incompatible water mix, the minerals can react and form solid deposits on the surfaces of pipes and other equipment.
A more detailed process of the reaction is illustrated here:
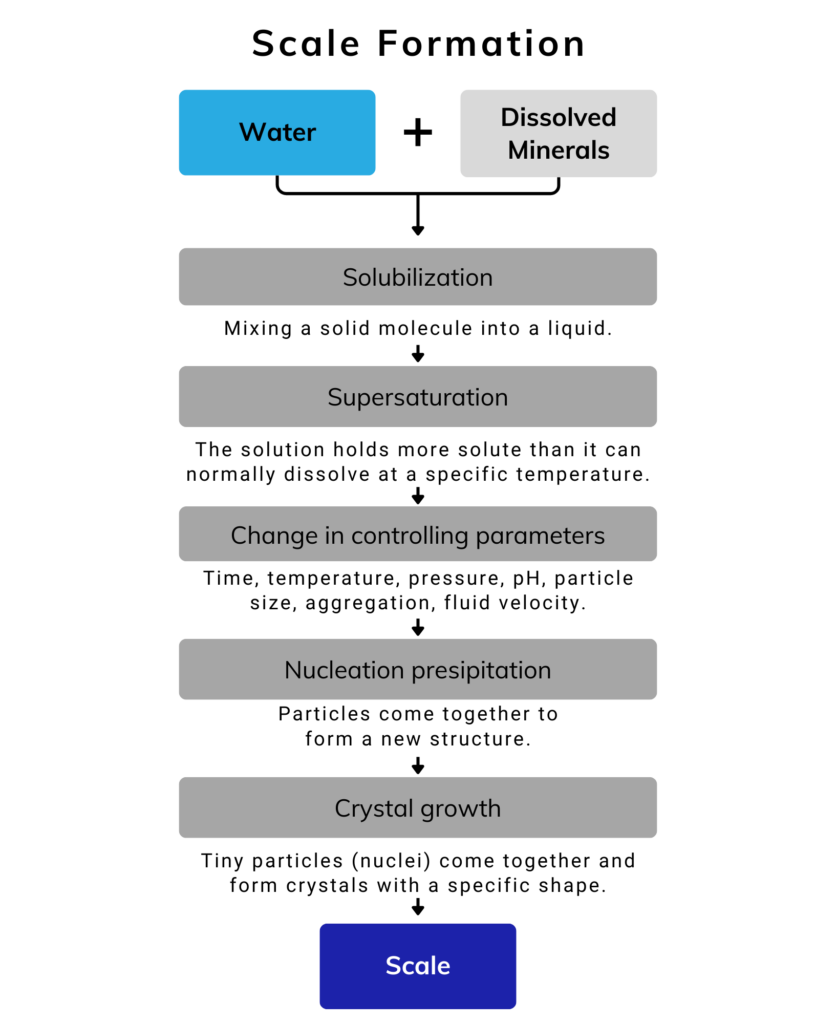
Common types of Scale
Calcium Carbonate (CaCO3):
- Formation: Calcium ions combine with carbonate ions in water.
- Contributing Factors: High pH levels, high temperatures, pressure changes.
- Removal: Treated with hydrochloric acid or organic acids.
- Example: Limescale on faucets in areas with high mineral content.
- Appearance: Typically white and chalky.


Calcium Sulfate (CaCO4):
- Formation: Reaction of calcium and sulfate ions, forming gypsum at temperatures below 200°F.
- Contributing Factors: High concentration of calcium and sulfate ions.
- Removal: Mechanical removal or acid treatment.
- Example: Found in reservoirs with high sulfate ion concentration.
- Appearance: Needle-shaped structure.
How can you tell the difference between CaCO3 and CaSO4?
CaCO3, or calcium carbonate, has a spiral growth and precipitates in the form of calcite. In contrast,
CaSO4, or calcium sulfate, precipitates in the form of gypsum and has a needle-shaped structure.

Naica Crystal Cave, a real mine in Chihuahua, Mexico has produced giant calcium sulfate scale, aka gypsum. Gypsum can form in pipelines in a similar chaotic structure albeit a lot smaller if the conditions are right.
Barium Sulfate (BaSO4) Scale:
- Formation: Barium ions in produced water react with sulfate ions.
- Contributing Factors: High concentrations of barium and sulfate ions, temperature, pressure, and pH changes.
- Removal: Difficult to remove; typically requires mechanical milling or specialized chemical treatments.
- Example: Prevalent in high-barium reservoirs.
- Appearance: Grayish, may have a blackish appearance due to impurities.
Strontium Sulfate (SrSO4) Scale:
- Formation: Strontium ions react with sulfate ions in produced water.
- Contributing Factors: Similar conditions to barium sulfate scale.
- Removal: Mechanical milling or chemical treatments.
- Example: Often found alongside barium sulfate scale.
- Appearance: Similar to barium sulfate, with a crystalline structure.

Iron Sulfide (FeS) Scale:
- Formation: Iron ions combine with sulfide ions, often in anaerobic conditions with sulfate-reducing bacteria.
- Contributing Factors: Corrosion, presence of sulfate-reducing bacteria.
- Removal: Chemical treatment, mechanical removal.
- Example: Found in pipelines prone to corrosion.
- Appearance: Dark, often black, resembling pyrite or Fool’s Gold.
Hydrate Scale:
- Formation: Solid crystalline compounds formed by water and gases like methane under specific temperature and pressure conditions.
- Contributing Factors: Low temperatures, high pressures in natural gas pipelines.
- Removal: Temperature and pressure adjustments, chemical inhibitors.
- Example: Blockages in natural gas pipelines.
- Appearance: Ice-like crystalline structure.
Asphaltene Scale:
- Formation: Solid crystalline compounds formed by water and gases like methane under specific temperature and pressure conditions.
- Contributing Factors: Low temperatures, high pressures in natural gas pipelines.
- Removal: Temperature and pressure adjustments, chemical inhibitors.
- Example: Blockages in natural gas pipelines.
- Appearance: Ice-like crystalline structure.
Silica Scale:
- Formation: Precipitation of dissolved silica in produced water.
- Contributing Factors: Temperature and pressure changes.
- Removal: Mechanical removal, chemical treatments.
- Example: Common in geothermal and some oilfield waters.
- Appearance: Hard, glassy deposits.
Paraffin Wax:
- Formation: Precipitates out of crude oil as it cools, aiding the formation of other scales.
- Contributing Factors: Lower temperatures in pipelines.
- Removal: Mechanical scraping, chemical solvents.
- Example: Common in crude oil pipelines exposed to cold environments.
- Appearance: Waxy, white or yellowish deposits.
Most scales encountered in the oil field are mixtures of two or more types. And as if that wasn’t enough, many other solids can be found in deposits, originating from the formation itself, completion materials, corrosion byproducts, and chemicals added during production or stimulation. In fact, ASTM lists over 120 different possible materials that can be found in water-formed deposits.
Understanding the basics of scale formation is just the beginning. In the next part of our series, we’ll explore the specific causes and detailed prevention and treatment strategies for scale in pipelines. Don’t miss Part 2 to learn more about how to keep your operations running smoothly and efficiently.
Continue to part 2.
Have questions about scale problems in your operations? Contact us to speak with an expert. Or return to the Q2 Technologies homepage to learn more about our solutions.







