Hydrogen sulfide (H2S) is a hazardous, corrosive, and toxic gas that can be found in oil and gas production. Its rotten egg smell is a distinctive marker and it is flammable. The smell can be detected at very low concentrations (about 1ppm), which serves as a warning as H2S is extremely toxic: at levels of 100ppm, inhalation of it can be fatal. In addition to being extremely toxic, H2S can cause severe corrosion issues to equipment. To avoid these operational risks and to meet commercial specifications, H2S must be removed from natural gas. According to general industry standards, gas is considered sour if it contains more than 4ppm of H2S.
What is MEA-Triazine?
There are many chemicals being used in the gas sweetening process, an MEA Triazine based H2S scavenger is one of the most common.
MEA Triazine (also known as Monoethanolamine Triazine, or simply just triazine) is a clear to light yellow liquid with a mild amine odor, which has a fishy smell. Triazine cannot be used in its pure form and so various concentrations of the product are manufactured, common field strength levels range from 20 to 80%. Its primary application is to remove hydrogen sulfide from gas and oil. Custom formulations based on this product can be blended with various polymers and additives to enhance or decrease certain attributes found in the natural gas stream.
Application Methods of MEA Triazine
Direct Injection, Contactor Tower
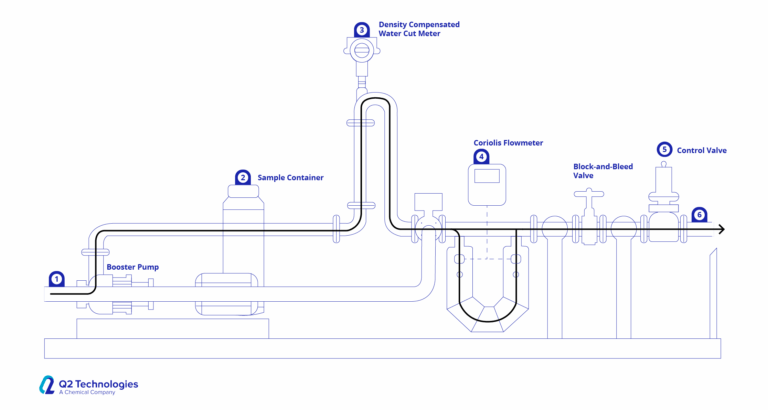
In many applications, triazine is injected directly into the gas stream. When there is good injection flow and enough time to respond, this approach works for H2S removal. Due to the H2S dissolving into the product, typical efficiencies are lower, although a removal efficiency of around 40% is to be expected. The location of the injection and the product choice must be carefully considered for direct injection to be successful. Atomizers, fog nozzles and static mixers tend to be used when applying MEA Triazine via direct injection.
Contactor Tower
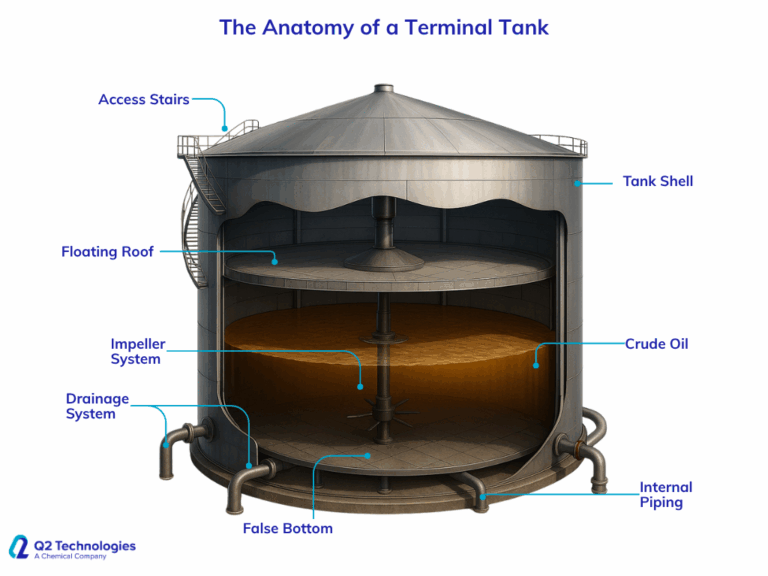
Another common approach is routing sour natural gas through a triazine-filled contactor tower. The tower layout can take on a variety of designs, but essentially H2S is eliminated when the gas passes through the liquid and dissolves into the triazine. The H2S removal efficiency of contactor towers can reach up to 80%. As a result, much less chemical is used, and OPEX may be significantly decreased. The contactor tower and chemical storage tanks, however, are less useful for offshore use since they take up a lot of room and weight.
Benefits of Q2 Technologies’ MEA Triazine
- Simple treatment with a low investment.
- When buying directly from the manufacturer you may reduce H2S scavenger costs.
- Up to 80% active triazine with other dilutions available.
- Over 20 years’ experience as Triazine manufacturers along plus applied engineering support.
- Turn-key automated skids for lease or purchase.
- Specialty blends with a variety of inhibitors.
Natural Gas Treatment Case Study
Case Study 305: Southeast Texas Results
Due to ongoing sulfur deposition and labor intensive change-outs, Q2 Technologies was invited to test our triazine process utilizing the same tower. Calculations indicated that a 50% reduction in total product would achieve the same results as the Sodium Nitrite. Five drums of triazine and five drums of fresh water were added to the contractor.
It was determined after the two-month test period, that our triazine achieved the same performance as Sulfa-Check with half as much product. In addition, the vessel was found to be free of solids upon inspection. There was no clean out involved and the reacted product was easily drained and the tower recharged.
Download this case study here.
Other Chemistries for H2S Removal
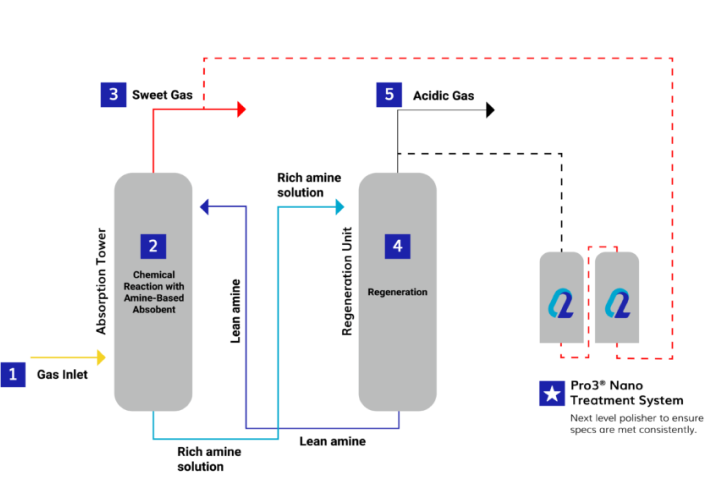
MEA-Triazine is an effective solution for H2S removal. However, each application is different and may require different scavenger alternatives. At Q2 Technologies, we also offer a non-triazine/non-amine H2S scavenger solution: Pro3® Nano. This alternative is a low CAPEX modular solution designed to lower LOE compared to conventional triazine scavengers. Learn more about the differences between Triazine and Non-Triazine for H2S Treatment and find the best solution for your operations. The Pro3® Nano chemical process is specifically designed to treat sour gas volumes using a unique combination of nano particles in a contact tower with a regenerative cycle.
What’s the best solution?
The best solution is whatever works best for your application. The main goal will always be H2S removal, but there are financial, operational, and commercial considerations that must be weighted.
- Have all KPIs (Key Performance Indicators) been identified and quantified?
- In doing so, are OPEX and CAPEX being optimized?
- Are the assets suffering from corrosion or over/under utilization?
- Is the hydrocarbon stream meeting commercial ppm requirements or thresholds?
These are some ideas to consider when choosing suitable treatment solutions, it not only depends on reaching H2S level requirements, but also on seeing how the scavenger affects your project as a whole. At Q2 Technologies we can help provide recommendations based on your specific needs, contact us and we’ll help you find exactly what you need to treat your sour gas.
Sources: